Despite the potential to be safer and cheaper than lithium-ion, aqueous batteries probably won’t wind up in your next EV. Here’s why.
Decarbonizing the energy and transport sectors requires the wide deployment of renewable energy and electric vehicles (EVs). Batteries are essential for both.
Today’s lithium-ion batteries are a good start, offering favorable energy density, cell voltage and lifespan. But the price, material availability and risk of thermal runaway of Li-ion batteries are downsides driving research into better alternatives.
Aqueous batteries are one such option. They’re safe, low cost, eco-friendly and have high ionic conductivity. However, their energy density and specific capacity are relatively low, limiting their practical use to large stationary energy storage where size is not an important factor.
What is an aqueous battery, and how do they differ from non-aqueous lithium-ion? Here’s everything you need to know about this promising energy technology.
Aqueous versus non-aqueous electrolytes
Aqueous batteries use water as the solvent for electrolytes. Traditional Li-ion batteries, in contrast, use non-aqueous carbonate and highly flammable organic solvent electrolytes. These electrolytes come with the risk of many serious hazards, such as harmful leakage, fire and explosion.
It is safer to replace these organic electrolytes with a non-volatile aqueous electrolyte with high thermal resistance. This would also lower the cost of the battery, as the separators and salts used for aqueous electrolytes are less expensive than for non-aqueous electrolytes.
However, because of their limited solubility and low cell voltage, aqueous batteries have a lower energy density than non-aqueous batteries. Aqueous electrolytes have high ionic conductivity, approximately 10-1 S/cm higher than the other types of electrolytes: organic electrolytes (10-3 – 10-2 S/cm), polymer electrolytes (10-7 – 10-3 S/cm) and inorganic solid electrolytes (10-7 – 10-2 S/cm).
|
Advantages of aqueous batteries |
Disadvantages of aqueous batteries |
|
High ion conductivity |
Instable SEI layer |
|
Thermal resistivity |
Narrow ESW |
|
Low cost |
Short cycling |
How to commercialize aqueous batteries
There are still challenges hampering the full commercialization of aqueous batteries. Aqueous electrolytes have a narrow electrochemical stability window, only 1.23 V, which is the main culprit for the decomposition of electrode materials. Researchers are looking for a suitable electrode material that can stay stable while operating in this condition.
An additional issue with aqueous batteries is their high self-discharging rate, where the battery loses its charge even while not in operation. This battery self-discharging is caused by the diffusion of ions through the electrolyte and the electrode materials’ reaction with water. With higher temperatures it becomes worse, causing corrosion of the electrodes and resulting in decreased battery performance and lifespan.
To enable aqueous batteries for demanding applications such as EVs or battery energy storage systems, the three most important parameters that must be improved are: higher cell voltage (which requires cathode improvements), a more stable metal anode interface and a larger electrolyte electrochemical window.
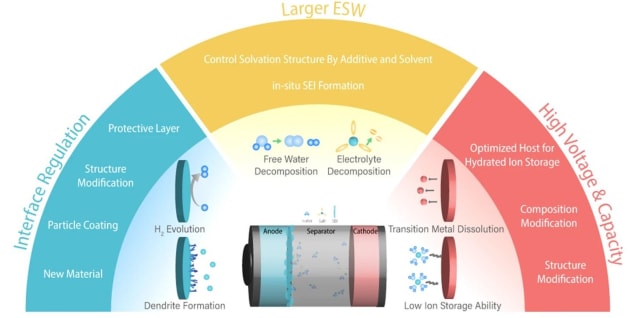
The electrochemical window of electrolytes is an important parameter for designing higher cell voltage batteries. At higher voltages, electrolytes decompose and interfere with the oxidation/reduction of the cathode/anode materials. The electrochemical window (EW) represents the range of the electrode’s electric potential between which the substance is neither oxidized nor reduced. The electrolytes should have a wider range of electrochemical windows that is greater than the range of the battery’s operating cell voltage. Electrolytes with narrow electrochemical windows are prone to irreversible decomposition, which deteriorates battery capacity. This affects the electrodes’ efficiency, since they react with the electrolyte instead of driving the electrochemical reaction.
Electrodes and electrolyte improvements for aqueous batteries
Researchers are exploring strategies to improve both electrodes and electrolytes to develop better aqueous batteries. To do so, they must find the proper electrode material and adapt the electrode’s structure to accelerate ion transport, improving interfacial stability and limiting the corrosion of current collectors.
Today’s commonly used Li-ion battery types are NMC (nickel manganese cobalt) and LFP (lithium iron phosphate). The LFP cathode is considered the most promising in organic electrolyte batteries. This is because of the wide availability of iron and the robustness of the phosphate anion, which make them safe for high-energy density and provide good performance of the battery. Unfortunately, well-performing cathodes that are proven in organic electrolytes may not provide the same performance in aqueous batteries. LFP cathodes cannot be used in aqueous electrolytes because of their surface instability that makes them electrochemically vulnerable.
Currently, the cathodes commonly used for aqueous batteries have a spinel structure (such as LMO, lithium-ion manganese oxide). The average cell voltage of spinel LMO is about 1.04 V, which is much lower than the LFP cell voltage of 3.2 V.
Researchers are looking to improve electrolytes by adjusting the composition of the solid electrolyte interphase (SEI) layer, extending the EW range and limiting water-based side reactions. The SEI is a passivation layer formed on the anode’s surface by electrolyte decomposition. The quality of the SEI plays a critical role in the long term cyclability and capacity of the battery.
The latest research in aqueous batteries
Researchers from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences recently developed an aqueous battery with significantly improved energy density. Published in Nature Energy, “Reversible multielectron transfer I−/IO3− cathode enabled by a hetero-halogen electrolyte for high-energy-density aqueous batteries,” documents the development of a multi-electron transfer battery that significantly improves energy density. The battery uses concentrated hetero-halogen electrolytes that contain ions from iodide (I–) and bromine (Br–) which enabled the multi-electron transfer. The battery used a metallic cadmium anode that provided a high energy density of over 1,200 Wh/L and a high current density of 120 mA/cm2.
The DICP researchers successfully demonstrated a safe and high energy density aqueous battery, an important step towards their use in demanding applications such as energy storage systems and possibly even EVs.